An ambitious vision:
From the outset, PROACT EU-Response has displayed an ambitious vision: to strengthen Europe’s capacity to respond effectively to future health emergencies by establishing a network of adaptive clinical trials capable of responding rapidly, ethically, and inclusively to emerging viral threats.
Today, PROACT EU-Response is entering its operational phase.
Governance structures are in place, scientific and clinical networks are active, and the methodological and social foundations for future trials have been laid.
This second article traces this progress, from vision to implementation, and outlines how PROACT EU-Response is gradually strengthening Europe’s preparedness capabilities through collaboration, innovation, and a patient-centered approach.
Laying the Foundations: Governance, Coordination, and Strategic Alignment
PROACT EU-Response is structured around strong coordination and shared responsibility within its consortium. The project is led by the Executive Committee, which brings together the heads of the various workstreams (i.e., thematic working groups responsible for different aspects of the project) and key partners to monitor and assess progress, ensure the coherence of activities, and anticipate next steps.
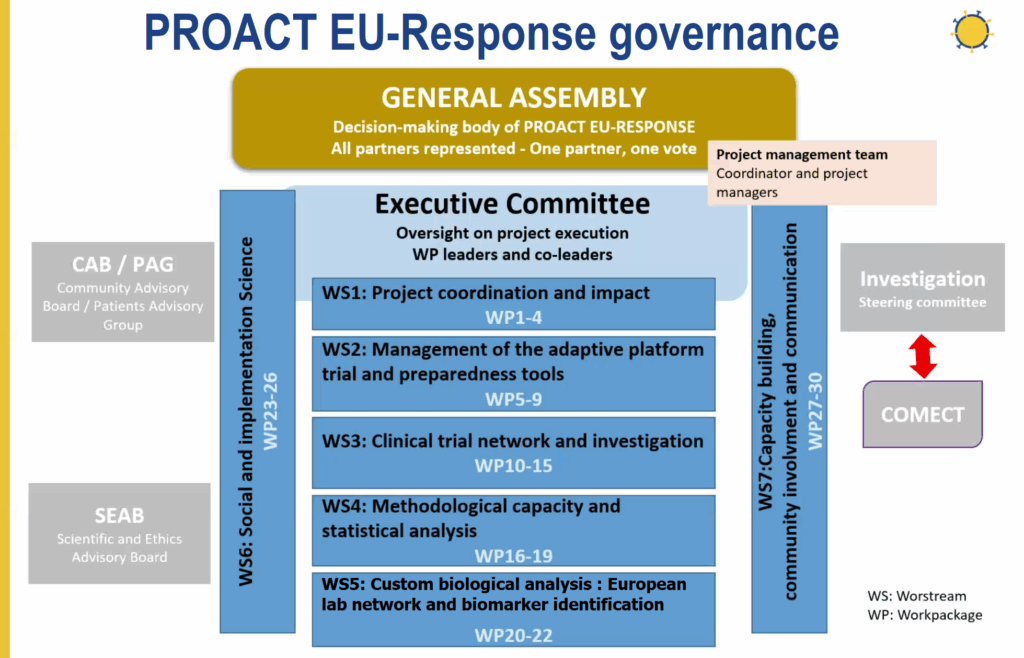
This governance framework enables the consortium to move forward in a coordinated manner across all scientific, clinical, and societal areas. Regular internal reporting cycles, shared documentation platforms, and structured evaluation processes ensure visibility of progress and harmonization of timelines.
This coordination is supported by partners, notably the European Patients’ Forum (EPF), which plays a central role in patient engagement, information dissemination, and external dialogue, as well as by academic and clinical institutions across Europe that anchor the project’s scientific and operational dimensions.
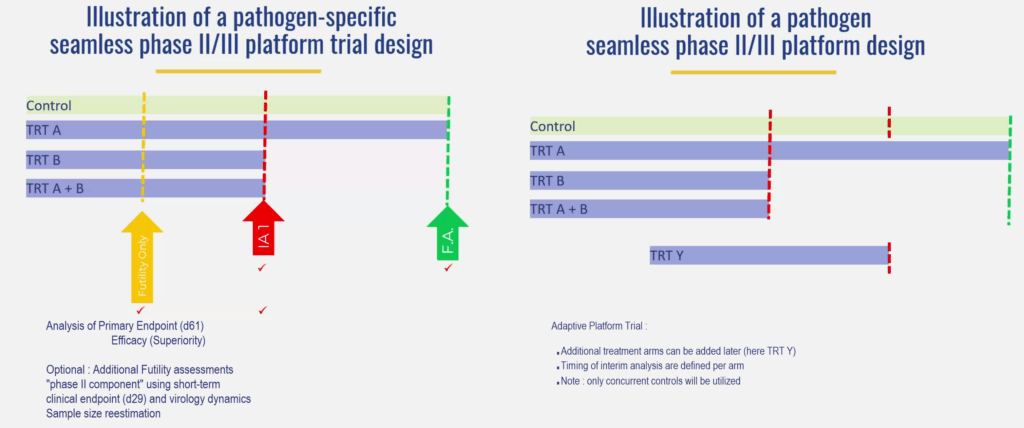
The adaptive trials at the heart of PROACT EU-Response are primarily based on the implementation of adaptive clinical trials (APTs) (i.e., trials that allow certain parts of the protocol, such as treatments or eligibility criteria, to be modified based on data collected during the study) and a trial architecture adapted to the volatile nature of respiratory virus epidemics.
Unlike conventional trials that evaluate a single intervention in isolation, adaptive platforms allow for the simultaneous evaluation of multiple therapies within a single, comprehensive protocol.
This design offers several advantages: New experimental therapies can be introduced as evidence emerges, the least effective treatment arms (i.e., groups of people receiving the same treatment) can be discontinued, and eligibility criteria can be refined over time.
The platform can operate for different pathogens and across different seasonal waves, thus ensuring continuity despite the evolving epidemiological situation (mutations, peaks, etc.). Recent internal work has clarified how this flexibility will be implemented in practice.
Some interventions, such as supportive or immunomodulatory treatments, may be used in all trial arms, while others will be evaluated in contexts specific to each pathogen, for example, for influenza or coronavirus infections.
Not all sites will activate all treatment arms continuously; participation will depend on local outbreaks, regulatory readiness, and feasibility. This pragmatic and modular approach ensures scientific rigor while preserving the agility needed to respond to health emergencies.
VIRvOLT: a European network of virology laboratories
Alongside the clinical network, VIRvOLT (Virology Operational Laboratories for Drug Testing), a European network of virology laboratories, ensures the reliability, comparability, and rapid availability of virology data generated during clinical trials. It is providing a robust infrastructure for monitoring antiviral efficacy across borders.
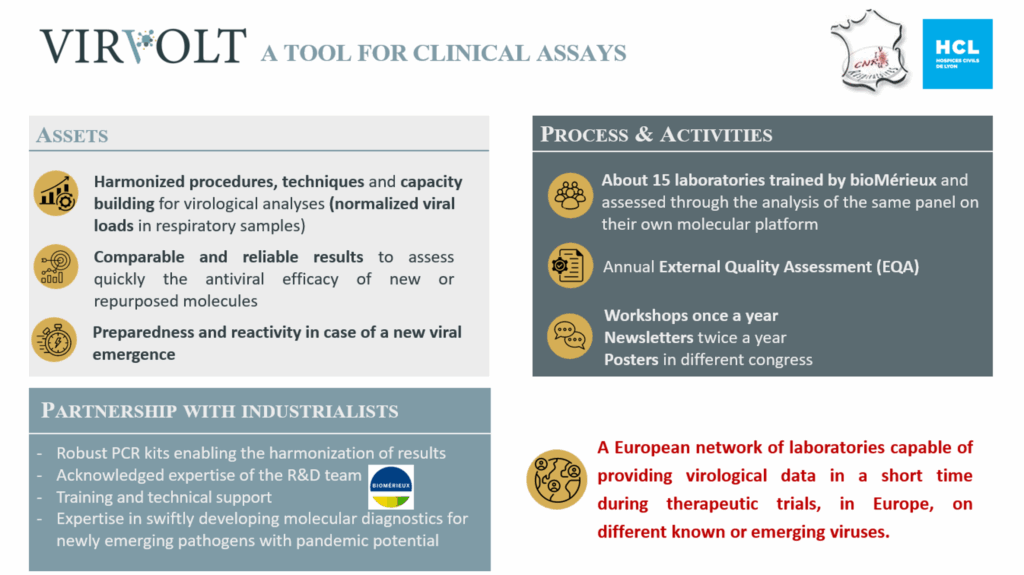
The laboratories participating in the VIRvOLT network, located in several European countries, have been trained and validated to apply standardized molecular techniques following the same protocol for comparable results between different laboratories using different platforms.
Partnerships with industrial players such as bioMérieux strengthen this ecosystem, enabling the rapid development and deployment of diagnostic tools for emerging pathogens.
In addition, the modelisation of these standardized virology data will contribute to increasing the statistical strength of clinicals trials, improving the data analysis, and contributing to the identification of more signals on drug antiviral activity.
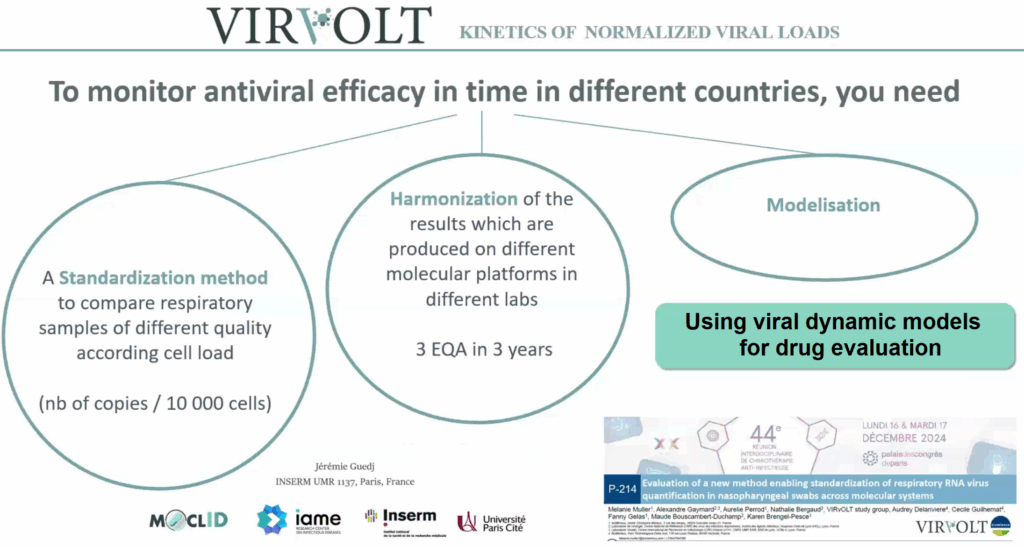
The laboratory network is designed to operate quickly. In the event of the emergence of a new respiratory virus with pandemic potential, the infrastructure is capable of providing standardized viral load data and comparable results across all sites within a very short time frame, enabling intermediate analyses and facilitating rapid decision-making.
Sample categorization and biobanking: Investing today to refine tomorrow’s knowledge
PROACT EU-Response takes a forward-looking approach to sample management, recognizing that clinical samples collected and analyzed today may form the basis for scientific breakthroughs tomorrow.
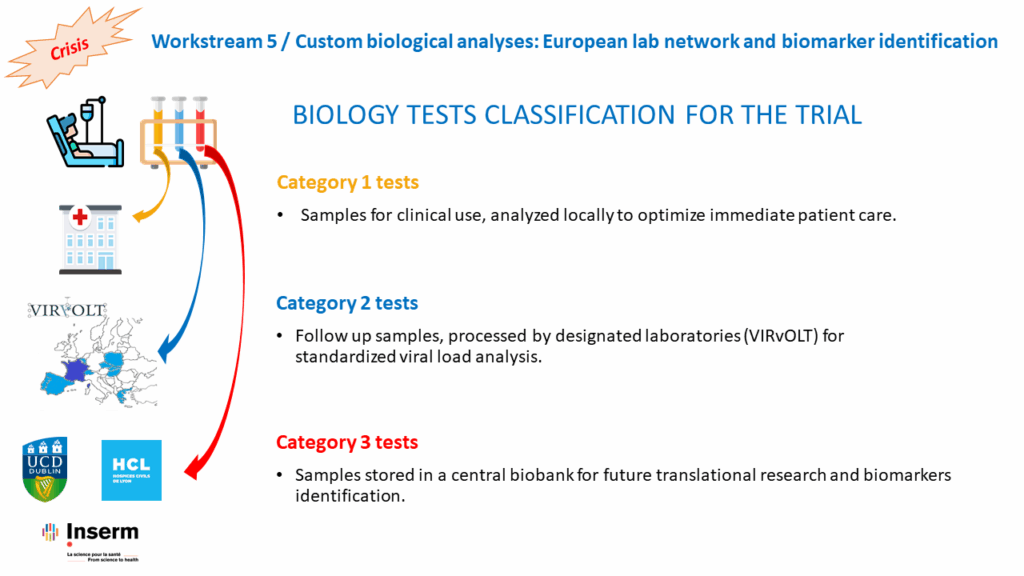
Samples collected during clinical trials are classified into three distinct categories.
This structure combines operational efficiency with long-term scientific value. The samples stored in biobanks will allow for exploratory analyses in immunology, host genetics, and metagenomics (i.e., the study of genetic content in samples from complex environments), enabling advancements in our understanding of disease mechanisms, treatment responses, and pathogen evolution.
By linking laboratory results to clinical and patient-reported data, the project aims to build a rich and multidimensional evidence base that goes far beyond the scope of a single trial or epidemic.
Strengthening evidence through target trial emulation
Alongside randomized controlled trials, PROACT EU-Response is also developing new methodological approaches that enable robust causal information to be extracted from observational data.
Increasingly recognized as an effective tool in epidemic research, target trial emulation (i.e., method consisting of simulating an ideal clinical trial using existing data to evaluate treatment effects) is one such new methodological approach.
Observational studies are often indispensable in emergency situations, but they are subject to confounding and other design-related biases related to their design.
Target trial emulation overcomes these difficulties by requiring researchers to explicitly define a hypothetical randomized trial, called a “target trial,” before analyzing observational data.
By aligning eligibility criteria, treatment strategies, follow-up periods, and outcomes with this protocol, analysts can significantly reduce bias and greatly improve transparency. Statistical techniques such as inverse probability weighting or propensity score matching (i.e., which aims to recreate the conditions of a clinical trial from observational data) are then applied to minimize residual confounding biases.
Within PROACT EU-Response, this framework is used to inform the selection and prioritization of antiviral candidates, thus complementing the evidence from adaptive trials and helping to guide strategic decisions regarding therapies to be evaluated later.
Ethics, regulation and cross-border consistency
The implementation of a multinational clinical research platform inevitably raises complex ethical and regulatory issues.
PROACT EU-Response addresses these issues through multi-level oversight and dedicated support structures.
The consortium established a Central Biobank to oversee and support the collection, processing, storage, and distribution of biological samples, ensuring their quality, integrity, and regulatory compliance.
Each participating site is supported to ensure compliance with national regulatory requirements, data protection rules, and ethics committee submission procedures, with the support of local clinical trial units.
At the project level, a steering committee and trial management team ensure scientific integrity, safety, and compliance throughout the trial lifecycle.
The consortium is also exploring solutions to further strengthen ethical oversight, with the aim of ensuring continuous, high-quality ethical review without compromising the speed and flexibility needed in crisis situations.
Inclusion as a scientific and ethical imperative
One of the project’s fundamental features is its commitment to inclusive research. Data from past pandemics have often underrepresented certain population groups, limiting the scope of the results.
To address this, trial protocols are designed to allow the inclusion of vulnerable and underrepresented groups, such as immunocompromised patients, pregnant women, and children, when scientifically and ethically feasible. Dialogue with regulatory authorities plays a central role in defining appropriate safeguards and inclusion criteria.
When direct inclusion is not immediately possible, exploratory substudies and parallel analyses are planned to generate safety and efficacy signals that can inform future trials.
Social and implementation sciences: Conducting effective clinical trials in real-world conditions
Recognizing that scientific excellence alone does not guarantee the success of clinical trials, PROACT EU-Response also integrates social and implementation sciences (i.e., studies on how trials are experienced, implemented, and perceived by staff and patients to improve their effectiveness) as a central pillar, through working groups dedicated to understanding the experience, implementation, and public perception of clinical trials.
Process evaluations are conducted to identify effective and less effective practices in real-world settings through analyses of barriers and facilitators to trial participation, organizational dynamics within hospitals, and feedback from patients and healthcare professionals.
The lessons learned are used to develop practical recommendations and tools to facilitate the design and implementation of inclusive clinical trials in hospitals. Knowledge sharing is actively encouraged within the consortium and beyond, so that the lessons learned benefit both PROACT EU-Response and the emergency preparedness ecosystem.
Communication, dissemination and patient involvement
With the launch of PROACT EU-Response, communication activities have moved from the design stage to implementation. The project website now contains articles, information about project members, the VIRvOLT virology network, and other structured content. A calendar of upcoming events allows readers to register for conferences.
The website also provides the public with access to documents and other content. The aim is to deliver clear and scientifically validated information about the project’s work and directions.
Content creation is increasingly collaborative. Working group leaders and communications officers regularly exchange and share information on scientific and methodological advances and outreach activities, ensuring that communications reflect the multidisciplinary nature of the project and remain high quality.
Patient mobilization is coordinated by the European Patients’ Forum (EPF) through a Patient Advisory Group (PAG), which contributes to strategic discussions, verifies the accessibility of documents, and ensures that patient perspectives are considered in decisions made within the various working groups. Capacity-building initiatives, including structured training, are planned to encourage the most meaningful participation possible.
Two major surveys are currently underway:
- A large-scale public survey conducted in eight European countries, exploring public perceptions of clinical research and willingness to participate in clinical trials.
- A complementary survey on misinformation, increasingly targeting journalists, institutional stakeholders, and social media users involved in health communication.
These surveys will enable the development of new strategies to improve public trust, combat misinformation more effectively, and adapt communication during future health crises.
PROACT EU-Response at the European Patients’ Forum Congress 2025
On November 26 and 27, the European Patients’ Forum Congress was held in Brussels, on the theme “Shaping the future of healthcare”.

PROACT EU-Response was presented during the plenary session “COVID-19, five years on: have our health systems recovered?”, which brought together policymakers, patient representatives, clinicians, and researchers to reflect on preparedness from a more global perspective.
EPF’s participation in the project was further developed, particularly regarding patient-centered preparedness, illustrating how social and implementation sciences contribute to more resilient and inclusive health systems.
Discussions focused on cross-border coordination, staffing challenges, regulatory decision-making in crisis situations, and the need for a more integrated European clinical trials infrastructure. The session also addressed future threats, including antimicrobial resistance, the burden of chronic diseases, and the health impacts of climate change.
PROACT EU-Response: From launch to deployment
As PROACT EU-Response enters a new phase, the focus is now on implementation and deployment. One area of work will focus on the transition to large-scale communication and dissemination activities, while fieldwork, investigations, site activation, and coordination between laboratories are intensifying.
A living infrastructure for health security in Europe
PROACT EU-Response now has a living infrastructure that brings together the scientific world and civil society through adaptive trial methodologies, clinical and laboratory networks across Europe, social sciences data, and patient involvement. Together, these elements form a complete and coherent whole, grounded in the desire to learn from past crises.
